Healthcare Professionals
NOTE: The information on this page is intended for healthcare professionals in the Canada.
MECHANISM
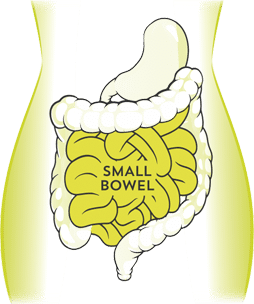
Atrantíl has a mechanism of action that relieves Irritable Bowel Syndrome (IBS) , Small Intestinal Bacterial Overgrowth (SIBO) ,bloating and abdominal discomfort accompanied by constipation, diarrhea or both by focusing on the duodenum.
Bacteria that are misplaced or overabundant in the small bowel produce free hydrogen as a byproduct, observed as undigested polysaccharide of carbohydrates.
Due to the proliferation of hydrogen, the intraluminal small bowel is an ideal site for archaebacteria and other anaerobic microorganisms.
Archaebacteria—a known methanogen in the human gastrointestinal tract—doesn’t belong in the small bowel. Methanogenesis occurs within the archaebacteria via reductase enzyme during cell metabolism, yielding methane as a byproduct.
Methane functions as a mechanical source of intraluminal distension, as well as an attenuator of regular motility.
Atrantíl is a unique, patented molecular combination of three natural botanical extracts. The first is a natural antispasmodic, which attenuates contractile responses to acetylcholine, histamine, 5-hydroxytryptamine and substance P.
By reducing calcium influx, the first botanical relaxes the gastrointestinal muscle and slows gastrointestinal motility in the duodenum.
This gives the other two botanical agents more time to act on bacteria and archaebacteria.
The second botanical extract—the flavonoid—begins to form covalent hydroxyl bonds and acts as a hydrogen sink, binding much of the free hydrogen available in the small bowel from misplaced bacteria. (The polyphenolic proanthocyanidins are naturally occurring and condensed. Having two aromatic rings connected by a three-carbon bridge lends in part to the macromolecule benefit of intraluminal action without systemic side effects.)
The archaebacteria require hydrogen for methane synthesis. By binding to the free hydrogen, the flavonoid removes much of the fuel source for methanogenesis.
In addition, the flavonoid binds to and weakens the cell wall of the archaebacteria, disrupting the integrity of the “S” layers of the cell wall making it more susceptible to Atrantíl’s third natural botanical extract.
This third botanical—a saponin/flavonoid—performs two functions. First, it kills off the bacteria in the duodenum.
The polycyclic peptide’s amino acids target receptors on the cell wall of the bacteria, and form pores by preventing cell wall synthesis resulting in lysing of the bacterial cell.
Second, the saponin/flavonoid penetrates the cell wall of the archaebacterial compromised by the flavonoid. Once inside of archaebacteria, the saponin/flavonoid binds to methyl-coenzyme M reductase halting methane production within the archaebacteria itself.
This halts methanogenesis, which reduces the presence of methane in the small bowel and alleviates discomfort caused by methane distension and constipation caused by methane non-absorption.
In summary, Atrantíl has three methods of action:
Through these three actions, Atrantíl returns the duodenum to its normal nearly sterile environment and enables the digestive tract to function normally.
Bloating and abdominal discomfort accompanied by constipation, diarrhea or both can be treated in the duodenum.
There are no known systemic side effects when utilizing Atrantíl therapy because its natural ingredients are macromolecules, which are intraluminal.
This allows the patient to benefit from a therapy that specifically targets the symptoms of Irritable Bowel Syndrome (IBS) , Small Intestinal Bacterial Overgrowth (SIBO) ,abdominal discomfort and bloating with or without constipation and diarrhea.
A SOLUTION THAT DOES MORE NATURALLY
We’ve formulated Atrantíl for patients who suffer from Irritable Bowel Syndrome (IBS) , Small Intestinal Bacterial Overgrowth (SIBO) ,bloating and abdominal discomfort accompanied by constipation, diarrhea or both. It’s an effective first-line treatment and a valuable therapeutic option for patients who haven’t found relief with other therapies.
Although Atrantíl has no known side effects, we haven’t tested it on pregnant or breast-feeding women. Therefore, we don’t recommend that pregnant or breast-feeding women take Atrantíl.
What should patients expect with Atrantíl
Atrantíl is formulated to address the root cause for Irritable Bowel Syndrome (IBS) , Small Intestinal Bacterial Overgrowth (SIBO), bloating and abdominal discomfort accompanied by constipation, diarrhea or both. For most patients, using Atrantíl will yield with the most favorable results realized through a regular dosing schedule.
Although there are no known side effects with Atrantíl, some patients may experience a Jarisch-Herxheimer reaction, also known as die-off.
Die-off is hypothesized to occur when certain bacteria are lysed and release low levels of an endotoxin.
This process can result in a brief period of low-grade fever, muscle aches or even diarrhea. When seen in patients, this die-off reaction is usually short in duration and a positive sign for several reasons.
The patient will appreciate your recommendation of a non-antibiotic, non-pharmaceutical treatment regimen.
RECOMMENDED DOSING
The time required to alleviate symptoms in patients taking Atrantíl will vary—dictated in large part by the amount of bacteria present. During clinical studies, some patients were symptom free in as few as two days.
Almost every patient found relief by day 10 of Atrantíl therapy with a dosing schedule of two capsules up to three times a day. Just as time to relief can vary, some patients will find control by adhering to a lower or less-frequent dosing schedule.
DOSING
Optimal results require compliance with dosing recommendations.
SAFETY AND EFFICACY
The patented formulation of Atrantíl comprises three natural botanicals. At the molecular level, Atrantíl’s action is intraluminal (minimally absorbed) due to the macromolecular structure.
The side effect profile for Atrantíl has been very minimal.
In a small number of patients, we have seen some mild abdominal discomfort when taken on an empty stomach.
Those symptoms resolved when taken with food. If the bacterial load is high, then a possible Herxheimer reaction (die-off reaction) may occur. This causes temporary symptoms such as muscle ache, foggy head, headache, etc.
This is believed to be a sign that Atrantíl is working and the reaction is related to the bad bacteria causing a reaction itself.
CLINICAL STUDIES AND RESULTS
In a double-blind study of patients complaining of bloating, constipation and abdominal discomfort, Atrantíl proved to be more than 88 percent effective in relieving those symptoms. The leading two prescription therapies did not perform as well vs. placebo according to their own studies.


In a double-blind study of patients complaining of bloating, constipation and abdominal discomfort, Atrantíl proved to be more than 88 percent effective in relieving those symptoms. The leading two prescription therapies did not perform as well vs. placebo according to their own studies.

To illustrate Atrantíl’s efficacy, the open-label trial targeted the toughest-to-address patients. Before beginning the Atrantíl trial, patients qualified only after failing to find relief from at least four pharmaceutical therapies.
The results demonstrated that 80 percent of the patients who had failed at other therapies found relief after using Atrantíl to control their bloating, constipation and abdominal discomfort.
Our clinical trial results have been published in the Journal of Gastroenterology and Hepatology Research and the World Journal of Gastrointestinal Pharmacology and Therapeutics.
FOR MORE INFORMATION
If you’re ready to give your patients the latest in efficacy and safety for Irritable Bowel Syndrome (IBS) , Small Intestinal Bacterial Overgrowth (SIBO), bloating and abdominal discomfort accompanied by constipation, diarrhea or both, the Atrantíl team’s here to help.
Request a physician kit now. And to schedule an in-person office visit or get answers to your questions, please contact:
Healthcare Concierge Hcp@atrantil.ca 214-984-3724 ext. 708